Metals and glass don't seem to have anything in common. Glass is generally transparent and fragile while metals are opaque and extremely strong; but under the right conditions, metals can form glass, and when they do, what results is an opaque, durable, scratch- and corrosion-resistant material that is often stronger than steel. Metallic glass is so versatile it can be used in iPhone cases, the lubricant-free gears of Moon rovers, and electrical transformers. Recently, experiments on the International Space Station that NASA’s Space Life and Physical Sciences Research and Applications (SLPSRA) division funded have revealed aspects of metallic glass formation that could open the door to even greater possibilities.
Current research in the field is focused on determining which metals form the best glass and how that glass can be used. The three most commonly used metals are zirconium, palladium, and iron, in that order, and the big questions in metallic glass research are:
- Will the metal turn into a glass?
- At what temperature will that transformation happen?
- What will the properties of the glass be?
- Can it be used for practical applications?
Until now, physicists tried to answer those questions by looking at what occurs at the temperature at which a molten metal actually transitions into a glass as it cools down. That transition point is called the glass transition temperature (Tg). What scientist Ken Kelton and his colleagues at Washington University at St. Louis discovered during their research project on the International Space Station is that the transition process actually starts while the metal is still a liquid, at a much higher temperature than Tg, and that by measuring how thick (viscous) the liquid is at that higher temperature, we can determine whether a glass will form and what some of its properties will be.
Kelton and his team performed two identical experiments that compared the temperatures at which liquid metals crystallized when they cooled down on the space station to the temperatures at which they crystallized when they cooled down on Earth. Gravity causes gases or liquids that are less dense than their surroundings to rise and those that are more dense to sink. This gentle motion creates what’s called gravitational stirring. That doesn’t happen in the microgravity environment of the space station, so the researchers wanted to see if the lack of gravitational stirring changed the liquids’ crystallization behavior. Microgravity makes it possible to measure things much more precisely than we can on Earth, so the team was confident any differences in the results would be accurate.
In addition, the team’s experiments were performed in the space station’s Electromagnetic Levitation Facility (EML), which suspends liquids in space using electromagnetic energy. EML facilities on Earth require so much power to break the hold of gravity and levitate the sample that they induce stirring. Recently, a feature was added to the space station EML that allows scientists to measure the electrical resistance of metallic liquids in the levitation chamber. No EML facility on Earth’s surface has this feature, and it made the most exciting findings of the team’s work possible.
The experiments produced two results the researchers didn't expect. The first is that the glass transition process starts far above Tg in liquid metals and the second is that, unlike other substances, above a certain temperature the electrical resistance in liquid metals doesn't increase when the temperature gets higher. Here's how that works.
Most of us are familiar with the fact that when you heat pancake syrup it becomes thinner and pours more easily. This occurs because heat makes the syrup molecules move faster. When the syrup cools, it becomes thicker because the molecules move more slowly. Electrical resistance operates something like this.
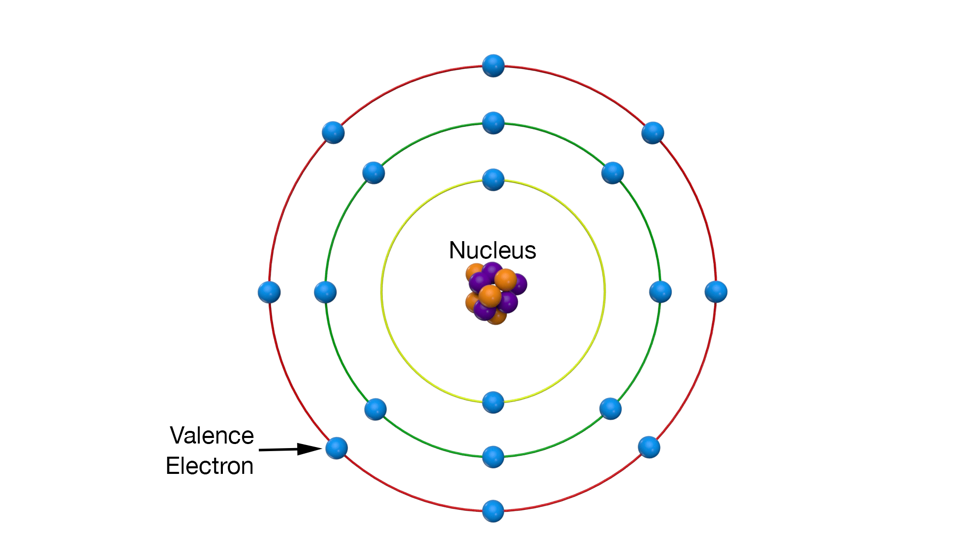
The classic model of an atom (Illustration) is a nucleus of protons and neutrons with electrons orbiting the nucleus. When an atom is on its own, all of its electrons stay with it; but in metals, whether they are solid or liquid, the outer set of electrons (called the valence electrons) merge together into what is called “the electron sea.” They are shared by all the other atoms and, when properly stimulated from the outside, form an electron stream or electrical current.
Think of a crowd of people. If they are standing still and you want to move through the crowd, it’s easy to do because you can walk in the spaces between the people. If they’re moving, however, it’s harder to get through the crowd because you can’t plan your route; and if they’re moving very fast, you start bumping into them and losing your balance. This is electrical resistance.
Atoms move faster when they’re heated, so an electron stream trying to get through a heated liquid or solid has a hard time because it keeps running into rapidly moving atoms. These collisions and impediments are what cause electrical resistance, and the higher the temperature, the more resistance there is.
What Kelton and his colleagues found was that, when some metal alloys are heated and become liquids, the electrical resistance doesn’t change much as the temperature increases. What surprised them was that, above certain temperatures, the electrical resistance of these metallic liquids doesn’t change at all! They don’t know why this happens, but they intend to find out. The answer could have a big impact on how we make things, especially things that have to withstand the extreme temperature swings and harsh conditions of space.
These two discoveries – that you can predict glass formation and type by measuring a molten metal's viscosity at temperatures far above Tg and that temperature doesn't affect electrical resistance at these temperatures – give physicists different places to set to work and a whole new set of phenomena to explore. Luckily for us, most of those phenomena will translate into practical applications that will make our lives easier. They may also translate into new materials that could improve long-term space travel. Who knows where we might end up when the universe is the limit.
Stay informed on other exciting SLPSRA research initiatives:
https://science.nasa.gov/biological-physical
For daily updates, follow @ISS_Research, Space Station Research and Technology News or our Facebook. Follow the ISS National Lab for information on its sponsored investigations. For opportunities to see the space station pass over your town, check out Spot the Station.