1 min read
Absorption and Emission Spectra of Various Elements
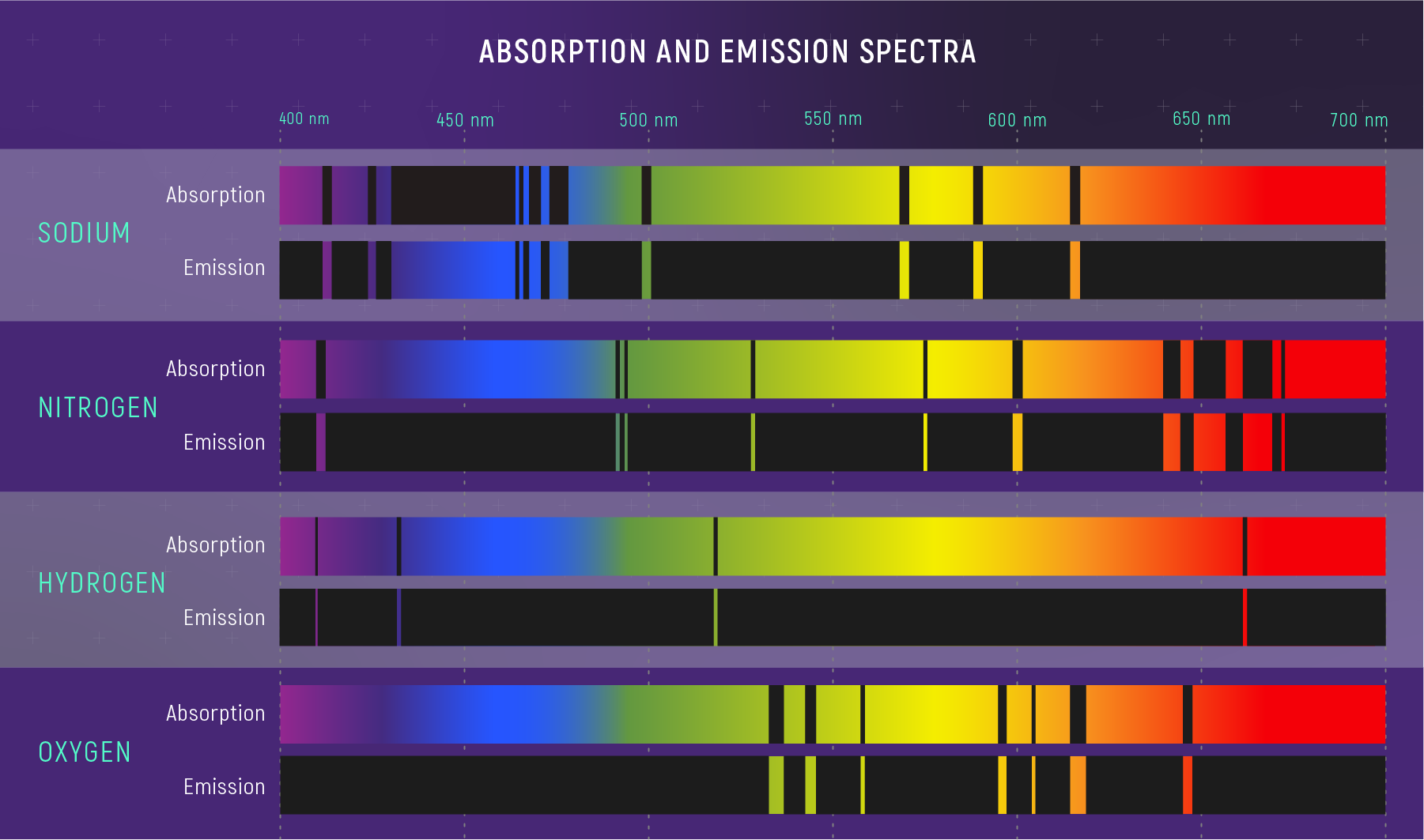
Absorption Spectra: When light passes through a gas, atoms and molecules in the gas absorb certain colors, or wavelengths, of that light. The result is an absorption spectrum: a rainbow with dark absorption lines.
Emission Spectra: The same gas can glow, giving off very specific colors to form an emission spectrum with bright lines known as emission lines.
Every element has a unique set of absorption and emission lines. The pattern of lines is known as a spectral signature. The absorption and emission spectra of each element are inverses of each other: The wavelengths of a particular element’s absorption lines are the same as the wavelengths of its emission lines. Astronomers can compare the spectrum of a celestial object or material with the spectra of known elements and molecules to figure out what the object or material is made of.
Share
Details
Laura Betz
NASA’s Goddard Space Flight Center
Greenbelt, Maryland
laura.e.betz@nasa.gov
NASA, ESA, CSA, Leah Hustak (STScI)