RTPCG-2
Science Objective
Phase II Real-time Protein Crystal Growth on Board the International Space Station (RTPCG-2) demonstrates new methods for producing high-quality protein crystals in microgravity. Previous work has shown that microgravity can sometimes produce high-quality protein crystals that can be analyzed to identify possible targets for drugs to treat disease. RTPCG-2 tests high-quality proteins for detailed analysis back on Earth.
Status
Planned for a future flight to the space station.
Experiment Description
The crystallization of macromolecules that produce crystals for structure determination has two distinct steps: 1) screening, and 2) optimization. In the first step, protein crystallization conditions are established by systematically screening a multitude of chemical and physical parameters. In the second step, the best conditions are optimized to produce crystals for structure determination. The last part of the final stage involves testing variations in concentration of precipitating agents.
Hanging-drop or sitting-drop vapor diffusion setups are the two most common methods used in the production of protein crystals. In the hanging-drop vapor diffusion crystal growth experiments, a small drop of protein (typically 4 to 8 mL) is placed on a flat surface (usually a microscope cover slip) and mixed with an equal volume of equilibrating solution. The drop is then suspended over a larger volume (usually 1 ml) of equilibrating solution (also referred to as “well” or “reservoir” solutions). The experimental chamber is sealed and allowed to equilibrate. The well solution contains a precipitating agent, usually a high concentration of an innocuous non-volatile chemical such as ammonium sulfate (AS) or polyethylene glycol (PEG).
In the hanging-drop process, the precipitation agent and protein concentrations have each been diluted in half by mixing the two volumes. During equilibration, water evaporates from both the drop and the well but accumulates in the well that has the higher concentration of precipitating agent. After equilibration, the drop and well solutions have approximately the same concentration of precipitating agent. As the protein slowly concentrates during the experiment, the solution becomes supersaturated since the volume is now shared with precipitating agent. The supersaturated protein solutions form crystal nuclei from which the larger crystals grow.
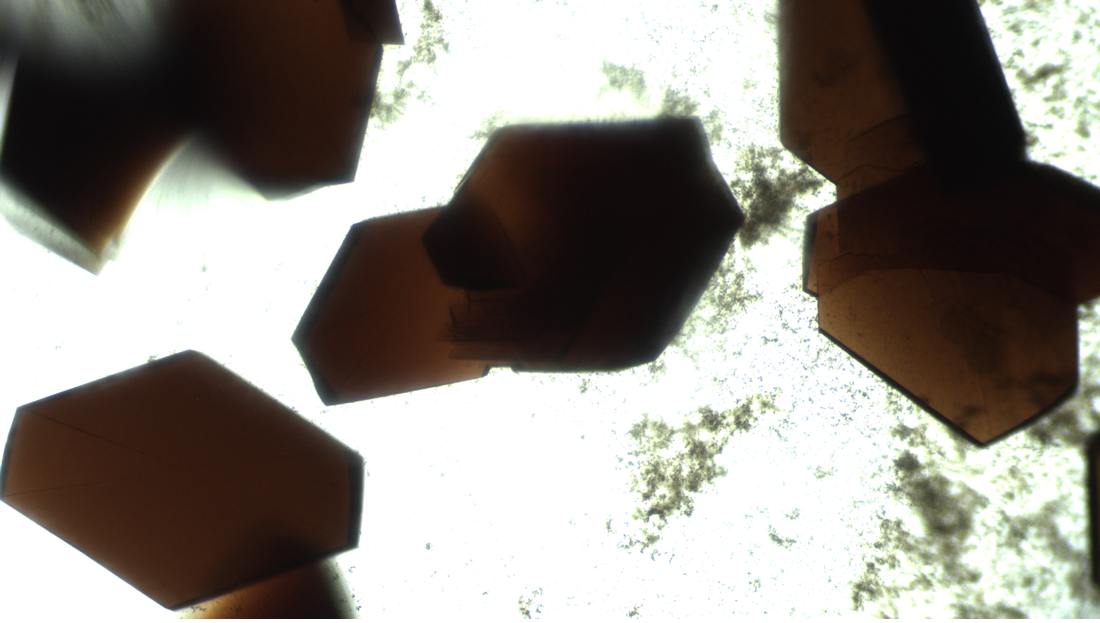
The Real Time Protein Crystal Growth (RTPCG) experiments are defined in a phased approach. Phase 2 investigates the nucleation, growth and crystallization of novel macromolecular crystallization targets in microgravity. The Phase II Real-time Protein Crystal Growth on Board the International Space Station (RTPCG-2) investigation uses EasyXtal hanging-drop vapor diffusion crystal plates (Fig. 1) and the sitting-drop New Century Pharmaceuticals Protein Crystallization Apparatus for Microgravity (PCAM, Fig. 2). These different plates are supported with kits that contain sealing strips for the PCAM plates, variable Ependorf pipettes (10, 100, and 1000 μl variable volume sizes), pipette tips, reagent solutions (protein solutions, precipitant buffers), containment and waste bags and plate setup support equipment.
The proteins to be studied have been selected by members of the RTPCG Science Definition Team (SDT). Four members of the SDT serve as individual Principal Investigators (PIs). Each PI investigates 2 proteins: a test protein with reasonably well-known and well-characterized protein crystal growth conditions and an experimental protein with more limited Earth-based crystallization success. RTPCG-2 evaluates 8 separate proteins in total.
BioServe Space Technologies (BioServe) serves as the implementation partner to plan, develop, and conduct the RTPCG-2 investigation. BioServe uses key hardware systems on orbit, specifically the Maintenance Work Area (MWA), BioServe microscope (Nikon TS-100 inverted microscope) and BioServe incubator (SABL) to meet the study requirements. During protein crystallization, which may require days or weeks, BioServe provides the use of SABL for precise temperature control and to protect the samples from disturbance. BioServe’s microscope is used for the observation of the protein crystals formed on-orbit and to document results using high-resolution imagery. Digital images are downlinked to Earth for evaluation by the project PIs.
On orbit, crew members pipette and locate fluids within specific wells (protein or reservoir) within the EasyXtall and PCAM plates. Protein droplet fluid volumes are very small, 2-50 μl and must be placed in the protein wells precisely. Because the volumes are small, the wells are sealed soon after loading to prevent evaporation. For the EasyXtal plates, a special hanging-drop lid (Drop Guard, Fig. 1B) seals onto each well. Microscope observation is done through this lid directly into the hanging protein drops. The sealing film used with the PCAM plate is transparent and also permits direct microscopy observations.