Identification of Biomarkers and Pathological Mechanisms via Longitudinal Analysis of Neurological and Cerebrovascular Responses to Neurotoxic Stress Using a Multi-cellular Integrated Model of the Human Brain
Science Objectives
Spaceflight and aging expose human brains to neurotoxic insults such as DNA damage, neuroinflammation, and the accumulation of pathogenic proteins such as amyloid, tau, and synuclein. In many individuals, these neurotoxic stresses diminish cognition by impairing critical processes such as learning and memory. However, cognition does not always decline uniformly with age or quantity of neurotoxic insults. Some individuals appear particularly vulnerable to stress-induced cognitive impairments while others are highly resilient. This investigation aims to develop technology to 1) determine how genetically diverse human brain tissue responds to neurotoxic stresses that may be encountered in space, 2) enable the discovery of prognostic biomarkers to forecast the probability of cognitive impairments, and 3) identify therapeutic means to prevent or reverse stress-induced neurodegeneration and cognitive impairment.
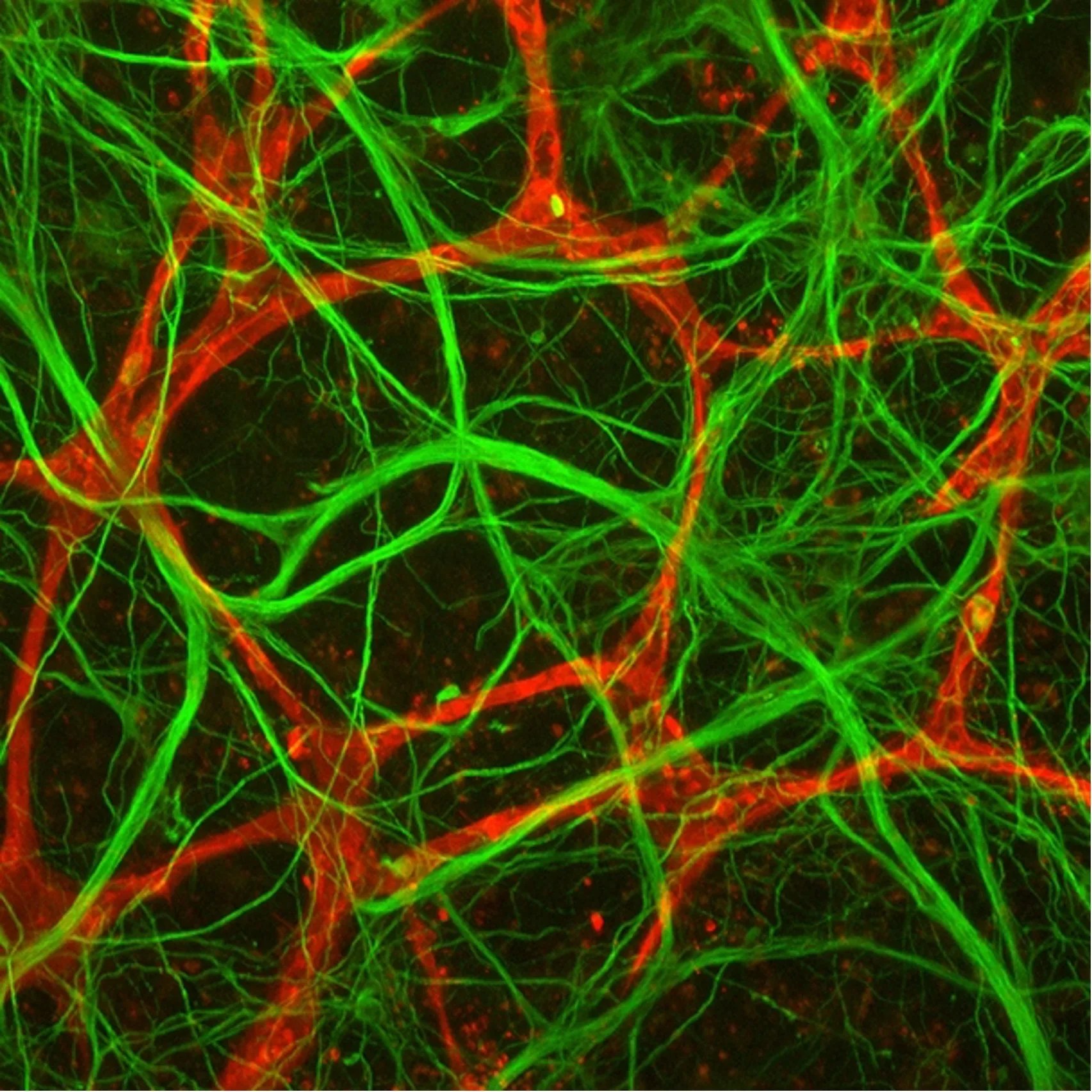
Experiment Description
It’s hypothesized that genetics influences how human brain tissues respond to neurotoxic stress, making some individuals more susceptible to cognitive Impairments. The Identification of Biomarkers and Pathological Mechanisms via Longitudinal Analysis of Neurological and Cerebrovascular Responses to Neurotoxic Stress Using a Multi-cellular Integrated Model of the Human Brain investigation will test this hypothesis by extending the longevity of the miBrain beyond 6-months, enabling longitudinal studies that map the response of human brain tissue to neurotoxic stress. Through state-of-the-art non-invasive high-content live imaging, researchers will track cells, tissues, health, and pathogenesis under multiple conditions, providing unprecedented insight into the dynamic changes that occur in human brain tissue following acute and chronic exposure to neurotoxic stress. This will allow researchers to develop a platform for assessing drug efficacy and toxicities across genetically diverse populations yielding insight into drug-genetic interactions that will inform and accelerate clinical translation.
Space Applications
An in vitro model of the human brain will be developed coupled with a comprehensive panel of non-invasive assays to longitudinally monitor the response of human brain tissue to neurotoxic stress for greater than 6 months. The enclosed and self-sufficient model of the human brain (miBrain) will allow studies modeling the effects of spaceflight on genetically diverse cohorts of human brain tissue. This will enable the development of prognostic indicators for potentially adverse neurological outcomes in space and highlight therapeutic strategies for mitigation.
Earth Applications
This study will pioneer a new technology to longitudinally monitor the response of human brain tissue to neurotoxic stress. This technology will be applied to discover genetic mechanisms that influence neurodegenerative processes in Alzheimer’s disease and related dementias that could be leveraged for intervention. This will establish a highly tractable model of the human brain that can be broadly applied across all neurological diseases to uncover new therapeutic opportunities.
Team
Principal Investigator
Joel W. Blanchard, PhD
Icahn School of Medicine at Mount Sinai, New York City, New York, United States
Co-Investigator
Woojin Han, PhD
Icahn School of Medicine at Mount Sinai, New York City, New York, United States




