Modeling Anthracycline-triggered Vascular Dysfunction
Science Objectives
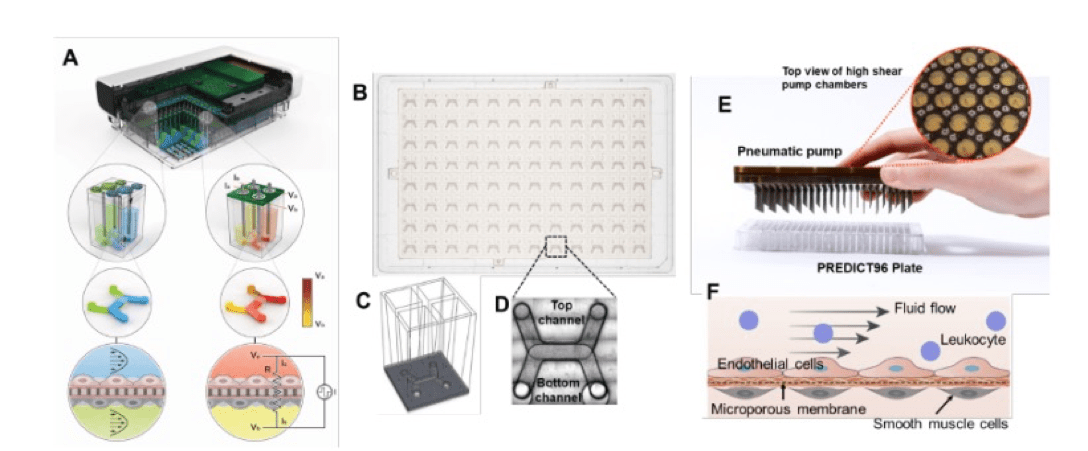
Cancer survival gains have revealed an unintended consequence of some therapies, an increased incidence of cardiovascular disease. Chemotherapy-induced vascular disease often occurs during active treatment, but it can have a delayed onset, too. The Modeling Anthracycline-triggered Vascular Dysfunction investigation seeks to use a well-established, first-of-its-kind high throughput organ-on-chip platform (Predict96) to extend the in vitro longevity of vascular tissues under flow to at least 6 months. This platform will be then used to mechanistically define how a commonly used chemotherapeutic agent for the treatment of breast cancer and childhood blood cancers affects the function of blood vessels with the goal of minimizing the vascular off-targets effect in cancer patients and survivors.
Experiment Description
The Modeling Anthracycline-triggered Vascular Dysfunction investigation seeks to study how doxorubicin acts as an acute and chronic stressor of vascular cells leading to changes in gene expression, global epigenetic states and functional phenotypes. In this investigation, previously unrecognized actions of the anthracycline and doxorubicin on vascular endothelial cells will provide the basis for establishing a novel conceptual and experimental framework seeking to model the long-term effects of doxorubicin in cells of the vascular wall. Researchers will adapt a now well-established, first-of-its-kind high throughput organ-on-chip platform (Predict96) to extend the in vitro longevity of vascular tissues to at least 6 months under flow conditions.
Recent advances in the development of complex human in vitro models have vastly expanded potential for biological and drug discovery. Nevertheless, current systems do not have the capability to assess the effects of acute and chronic stressors on cellular systems for long periods of time. This feature is critical to model the effects of certain stressors on relevant target tissues. An important class of such stressors are chemotherapeutic agents currently in use for the treatment of cancer. In particular, the use of anthracyclines, a class of chemotherapeutic drugs, is associated with clinical heart failure and other cardiovascular pathologies including hypertension, vascular inflammation, and ischemic heart disease. The mechanisms underlying these adverse effects of anthracyclines on the vasculature have not yet been fully elucidated.
Space Applications
New findings derived from this project could be very valuable in the design and implementation of medical treatments for astronauts participating in long-term space missions.
Earth Applications
These studies should define the basis for establishing novel conceptual and experimental frameworks to study the effects of chemotherapeutic agents on the human vascular system. The results should also mechanistically define how doxorubicin triggers vascular dysfunction which will help in the development on new therapeutic interventions to curtail these off-target effects.
Team
Principal Investigator
Guillermo Garcia-Cardena, Ph.D.
Brigham and Women’s Hospital, Boston, Massachusetts, United States
Co-Investigator
Corin Williams, Ph.D.
Draper Laboratory, Cambridge, Massachusetts, United States
Hesham Azizgoshani, Ph.D.
Draper Laboratory, Cambridge, Massachusetts, United States